queequeg152 said:
eh, i promise im not stalking you. i seem to be posting in all of your threads recently tho. our interests must intersect.
that pen is very cool. a portable based Chlorine generator means all you need to haul along is salt, that's brilliant.
what is this product exactly tho( the clear rez) ? im confused, you say its not bleach, yet you say its HOCl?
FWIW i have lots of experiance with pools and related knowlage (had a pool when i was young), so im very familiar with salt chlorine generators, as well as chemistry in general.
the biggest problem i see here is the need for vast amounts of salt to be in solution in order for the generator to operate efficiently. i believe you need something like 2000+ ppm NaCl.
This concentration would kill plants.
however how does this pen work? i imagine you don't want to drink salt water right? so it must be able to isolate the salt water solution from the drinking water?
if this is the case, then how could you adapt it for use in a recirculating system? you would need to either use it batch wise, or set it up to constantly regenerate the salt solution.
i have always been an advocate for chlorine disinfection, big time. its superior in almost all ways. the best thing imo is the ease at which you can test the chlorine concentration... With 5cent per shot pool DPD test kits.
with that said, idk if chlorine generators make the most sense, considering that you can buy what i call "floaters", designed for like 500gallon spas that use 1.25" chlorine pucks. one of these set up to very very slowly leach chlorine into your system would work fantastically IMO.
TBH tho i have not tried this myself. I invested in solenoid dosing pumps, and tbh i now think they are over kill, but i have them none the less, so i dose via bleach solution, if i do dose at all.
i do think floaters would be superior tho. from the cost standpoint.
Very very large nurseries and greenhouses tend to use either ozone(organic places) or chlorine dioxide ejectors
(very very cost effective way to dose Hocl, but extremly dangerous if mishandled...
ive never heard of chlorine generators in use in any commercial operations.
I know your not stalking me. I'm just the Mac Gyver type probably like you.
The Clear Rez is a product the eZCloner people market.
http://www.ezclone.com/our-products/solutions/clear-rez-2/
It works really well but can be pricey. If you look at the MSDS sheet it is a small amount of HOCL it contains. Less then 1%. HOCL is very powerful. Kind of like hydrogen peroxide, but better. Both are oxidants.
For pools you need a lot of salt because of the high surface area, changing weather (heat), sunlight breakdown, etc of the HOCL.
With this pen it is ment to sterilize the water and then drink it almost immediately. Or you usually store it in a sealed container. The pool need lots of spare HOCL to kill anything introduced into it. You drinking water is good once done. Hence less salt.
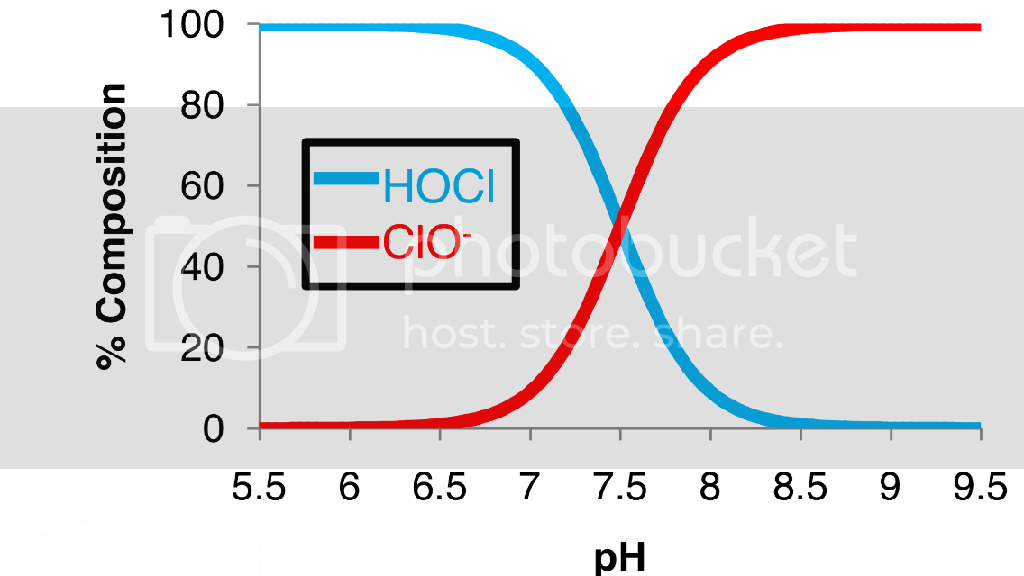
HOCL in high concentrations breaks down very fast, in a matter of 30 minutes to 4 hours from what I have read. The less concentration the more stable it is. Also the PH breaks it down very fast. It wants to be at about 5 to 6.5 PH to be stable.
We all know bleach is way above 5 PH. HOCL is a small active ingredient in bleach. So for bleach they add lye to stabalize the HOCL.
If you can get almost pure HOCL it kills most stuff on contact within seconds, and then breaks down rapidly. Hospitals are looking at this to sterilize rooms because of crazy staph that can't be treated be antibiotics. HOCL kills even this staph.
Military has been using this pen for a while. They are looking for something that can be used in a Nuclear Biological contamination scenario. This pen wouldn't qualify. They are looking at a camel-bak type pouch with this pen built into the lid.
http://ciehub.info/References/www.marcorsyscom.usmc.mil/sites/pmice/InfoPapers/Armor/IWPS.pdf
If you can generate your own HOCL close to you source of water you don't need as much salt, and HOCL is more concentrated.
I will generate some on a small scale to try. My one test hydro bed only has a 3 gallon res. 4 gallons if topped off all the way.
HOCL also cuts thru and kills bio-film. The slime you get on roots. That is why Clear Rez has been such a break thru. No just bleach, but HOCL in concentrate.
I have had people tell me Clear Rez is the same as bleach. Really Clear Rez is PH 5 to 6.5 and bleach is usually around 12. Defiantly not the same. Maybe the same active ingredient, plus it's where you want your water 5.5 PH for hydro and nutes. People think they can just dilute down bleach to get HOCL equivilant. It is close, but bleach has other bad things in it are not good for plants, ex lye.
Why is bleach usually off yellow? I think pure HOCL is crystal clear.
Why not just use or make HOCL for your system? And it will be happy at hydro PH levels.
I too cleaned pools in my younger years. Way before salt water pools. Measuring and dosing the water to get it in balance...days of holes burned in Levi's from chlorine and acid.
Here is another interesting article I found about chlorine and PH...
http://www.nsf.org/newsroom_pdf/articles_washwater.pdf
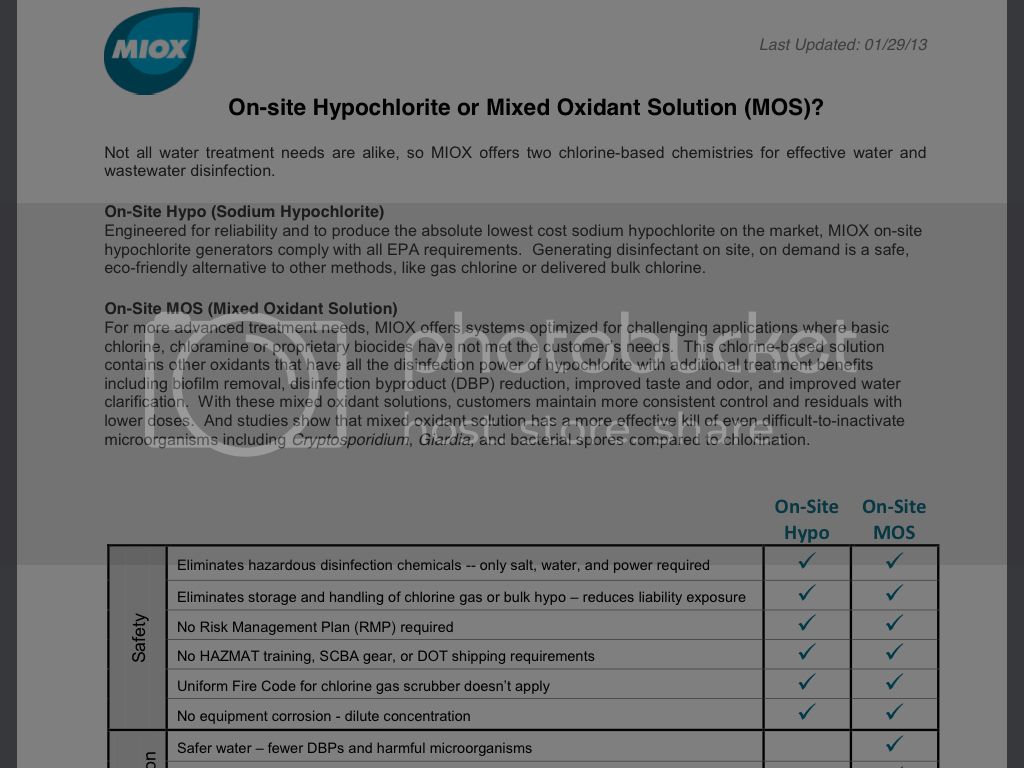
MIOX.com of stats...