-
If you have a question about commercial production or the hot sauce business, please post in Sauce Biz.
-
✅ Expert and friendly hot pepper grow advice.
✅ The latest information on hot pepper varieties.
✅ Reliable seed trading.
✅ Hot sauce recipes and food safety guidance.
✅ Hot sauce business tips for startups.
🌶️ And more!
It's all here, at The Hot Pepper! The Internet's original hot pepper community! Est. 2004.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
food-safety pH is a log scale - are we using too much vinegar?
- Thread starter Siv
- Start date
The problem here is the separation not the acidity per se. When it separates you no longer have uniform acid, you have 2 zones of acidity, so one was too low (in acid) and went bad. This can even affect commercial sauces, ones you have to give a shake. An emulsifier like xanthan is recommended if you have a separation issue.So I thought it important to update this. Oak milk is a no no for hot sauce!
I was happily eating the sauce for the first few weeks after making it but then it got lost in the rotation and I forgot about it. The mason jar load was kept in the fridge and I opened it the other day. The "sauce" had separated - there was a clear liquid on top with gloop on the bottom. I sniffed and the liquid smelled off. I poured it down the sink - as I was pouring the gloop at the bottom smelled great but I wasn't brave enough to try it. In hindsight I should have checked the pH of the liquid - I would have imagined it stayed pretty acidic so I'm surprised that it went off. Anyway, here ends my oat milk sauce making antics!
The problem here is the separation not the acidity per se. When it separates you no longer have uniform acid, you have 2 zones of acidity, so one was too low (in acid) and went bad. This can even affect commercial sauces, ones you have to give a shake. An emulsifier like xanthan is recommended if you have a separation issue.
 GASP!!! You said the X- word!!!
GASP!!! You said the X- word!!! Interesting thoughts.
My thought is, although there may be separate layers, with one having more vegetable matter and the other more liquids/acids, the vegetable matter should be acidified and therefore safe. Like a cucumber after being soaked in vinegar, it is acidified.
This comes back to "finished equilibrium ". When testing a sauce for pH, my process authority says to measure pH 24 hours after blending/processing. It allows the vinegar to permeate all of the vegetable matter. If the veg pieces are really small...like in a blendered sauce...the vinegar will permeate quickly. If the veg is larger...like a pickle spear... it may take up to 2 weeks for the vinegar to permeate.
Yes but commerical oat milk has oil added. Oils, as you know, are not water soluble so must be emulsified to gain the benefit of a properly balanced sauce (pH) since they can harbor other ingredients from the sauce including the oats. Which, most likely, went bad (about the same time the oat milk would in the fridge) since it separated from the sauce.
I bet that oat milk had some kind of vegetable oil added. You could try Planet Oat which has no oils. It also has gellan gum. It might emulsify w/o an additional emulsifier since it's sans oil.
I'm just wingin it so....Going with what Boss said. ^^^
Seriously, your posts hold way more weight than mine. However I would question any oil (sunflower etc.) that is added to oat milk.
I see then it might just be a basic pH issue.
I have xanthan but always forget to use it. I got a little worried about this whole separation thing as I have a lot of sauces that separate. I fount this stash from last year up on the shelf - they really don't look that great!

But I cracked open the bottles and they smelled fine - not just fine but good enough to have me salivating!
So I took a pippette and siphoned off the top liquid from each bottle and checked pH:
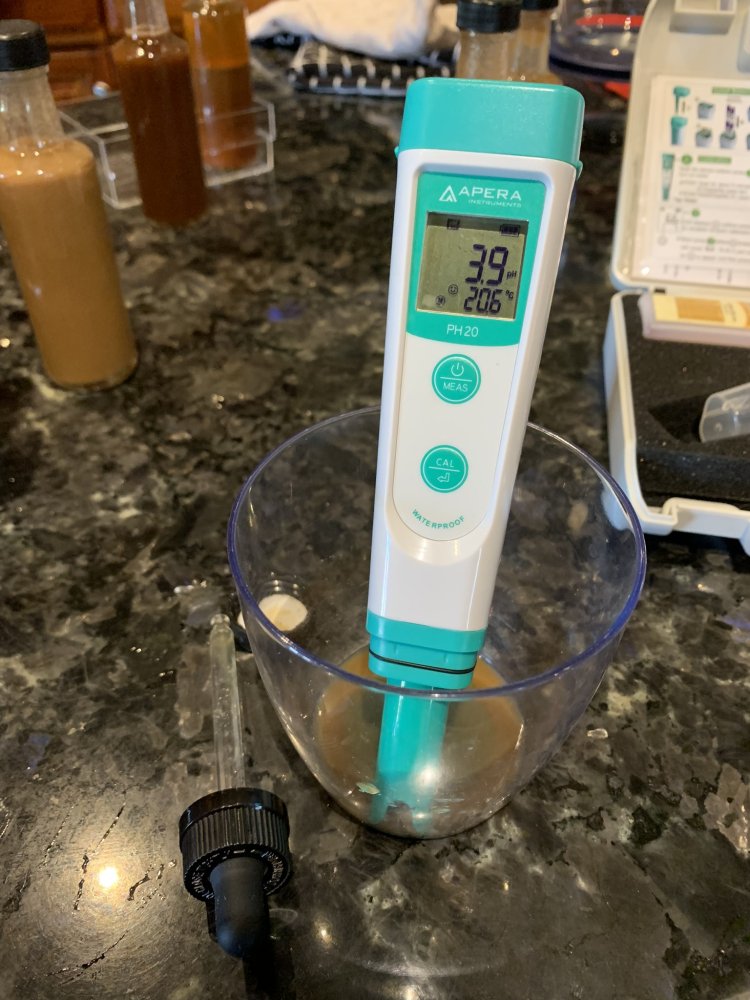
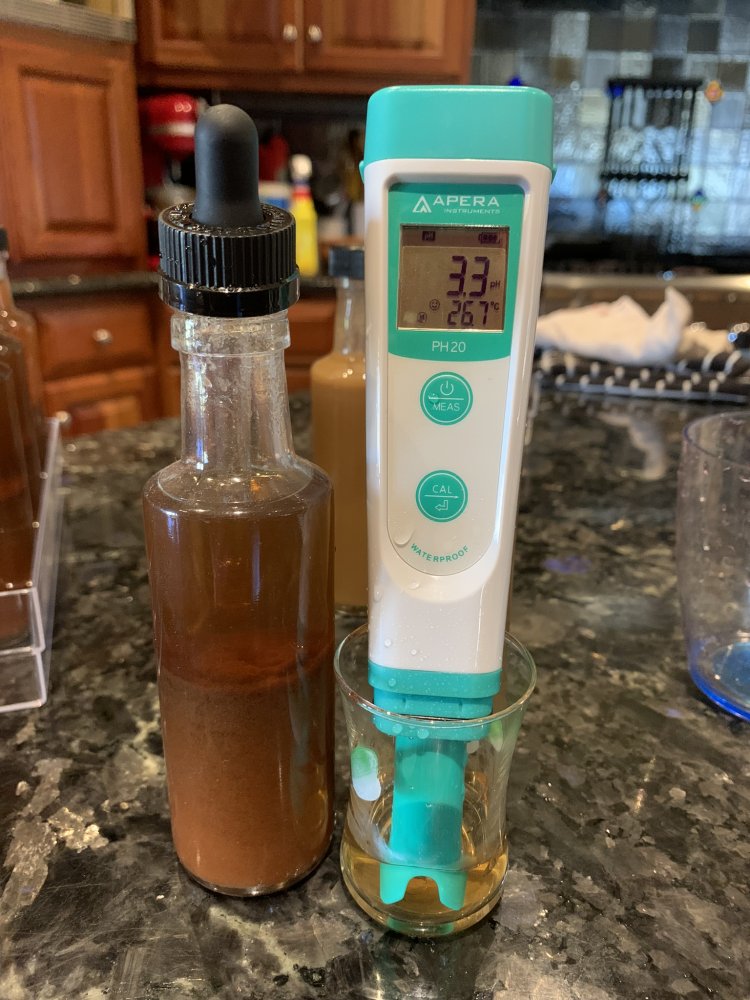
Both are well below 4. A little shake and things are looking normal:

Now I'm toying with the idea of just acidifying some oat milk, sticking it in a bottle and leaving it to separate as an experiment.
But I cracked open the bottles and they smelled fine - not just fine but good enough to have me salivating!
So I took a pippette and siphoned off the top liquid from each bottle and checked pH:
Both are well below 4. A little shake and things are looking normal:
Now I'm toying with the idea of just acidifying some oat milk, sticking it in a bottle and leaving it to separate as an experiment.
Last edited:
I wish you could have checked the pH of the bottom liquid as well. And then the shaken. Just to check this theory. Like maybe the more acidic could even be the top (vinegar which is water) when there are no fats.
Next experiment: Test the two zones and the unified zone. 
